TheDexterians
Blood is our Gold
Causes, Signs, and Symptoms
The most common and best-known type of sickle cell disease is sickle cell anemia, which is also called meniscocytosis, sicklemia, or SS disease. All types of sickle cell disease are caused by a genetic change in hemoglobin, the oxygen-carrying protein inside the red blood cells. The red blood cells of affected individuals contain a predominance of a structural variant of the usual adult hemoglobin. This variant hemoglobin, called sickle hemoglobin, has a tendency to polymerize into rod-like structures that alter the shape of the usually flexible red blood cells. The cells take on a shape that resembles a curved blade. Sickle cells have a shorter lifespan than normal shaped red blood cells. This results in chronic anemia characterized by low levels of hemoglobin and decreased numbers of red blood cells. Sickle cells are also less flexible and stickier than normal red blood cells, and can become trapped in small blood vessels preventing blood flow. This compromises the delivery of oxygen, which can result in pain and damage to associated tissues and organs.
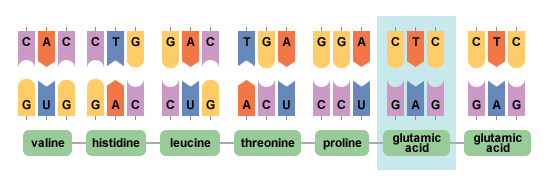
Humans normally make several types of the oxygen-carrying protein hemoglobin. An individual's stage in development determines whether he or she makes primarily embryonic, fetal, or adult hemoglobins. All types of hemoglobin are made of three components: heme, alpha globin, and beta globin. Sickle hemoglobin is the result of a genetic change in the beta globin component of normal adult hemoglobin. The beta globin gene is located on chromosome 11. The sickle cell form of the beta globin gene results from the substitution of a single DNA nucleotide, or genetic building-block. The change from adenine to thymine at codon (position) 6 of the beta globin gene leads to insertion of the amino acid valine-instead of glutamic acid—at this same position in the beta globin protein. As a result of this change, sickle hemoglobin has unique properties in comparison to the usual type of adult hemoglobin.
Sickle cell anemia is caused by a point mutation in the beta-globin chain of hemoglobin, causing the hydrophilic amino acid glutamic acid to be replaced with the hydrophobic amino acid valine at the sixth position. The beta-globin gene is found on the short arm of chromosome 11. Under low-oxygen conditions the absence of a polar amino acid at position six of the beta-globin chain promotes the non-covalent polymerisation of hemoglobin, which distorts red blood cells into a sickle shape and decreases their elasticity.
Sequence for Normal Hemoglobin
ATG GTGCACCTGACTCCTGAGGAGAAGTCTGCCGTTACT
START ValHisLeuThrProGluGluLysSerAlaValThr
Sequence for Sickle Cell Hemoglobin
ATG GTGCACCTGACTCCTGtGGAGAAGTCTGCCGTTACT
START ValHisLeuThrProValGluLysSerAlaValThr
References:
Mutation. (n.d.). BBC News. Retrieved May 1, 2014, from http://www.bbc.co.uk/bitesize/higher/biology/genetics_adaptation/mutations/revision/1/
Sickle cell disease. (n.d.). - Genetics Home Reference. Retrieved April 30, 2014, from http://ghr.nlm.nih.gov/condition/sickle-cell-disease